- Dr. Elad Levy, Co-Principal Investigator of the COMMAND trial awarded the Duke Samson Award for the upcoming results presentation on September 30, 2024
Synchron Announces COMMAND Study Results at the 2024 Congress of Neurological Surgeons Annual Meeting
Media
Kimberly Ha
Synchron
kha@synchron.com
Synchron, the brain-computer interface (BCI) company, today announced the presentation of its COMMAND study results at the 2024 Congress of Neurological Surgeons (CNS) Annual Meeting, held September 28 – October 2, 2024, in Houston, Texas.
Dr. Elad Levy, one of the Principal Investigators of the COMMAND trial, will present the study results, and has been honored with the Duke Samson Award for his abstract on the trial results.
The presentation will highlight early feasibility data from the US-based COMMAND trial, where the primary goal of the study assessed the safety of the neurointerventional procedure to implant the Stentrode into the blood vessels of the brain. The study focuses on Synchron's BCI technology aimed at improving functional independence for patients with debilitating neurological conditions.
CNS 2024 Presentation Details:
Title: Results of the COMMAND Trial: An Early Feasibility Study of the Synchron Endovascular Brain-Computer Interface
Session: General Scientific Session II
Date/Time: Monday, September 30, 2024: 10:51 – 10:59 a.m. CDT
Presenter: Elad I. Levy, M.D., MBA, FAANS, FACS, Principal Investigator, COMMAND trial, and Professor of Neurosurgery, State University of New York at Buffalo.
The abstract is available on the CNS website. The presentation will also be accessible on the Media page of Synchron’s website at www.synchron.com.
“We’d like to congratulate Dr. Levy for his achievements and the recognition from the neurosurgeon community with the Duke Samson Award,” said Tom Oxley, M.D., Ph.D., CEO & Founder, Synchron. “The COMMAND trial results are a significant milestone in our efforts to transform the lives of patients with neurological disorders, and we look forward to sharing our data for the first time at CNS 2024.”
“It is an honor to present Synchron’s COMMAND study results and be recognized with the Duke Samson Award. Synchron’s technology has the potential to improve the lives of millions of patients with paralysis and other neurological disorders, and I am excited to share our progress at the CNS Annual Meeting,” said Levy.
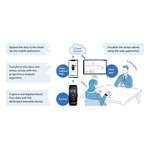
The COMMAND trial enrolled six patients and is being conducted under the first investigational device exemption (IDE) awarded by the FDA to a company assessing a permanently implanted BCI. The early feasibility study primarily assesses safety while also evaluating quantified efficacy measures of the Synchron BCI motor neuroprosthesis in patients with severe paralysis. The total number of people implanted with a Stentrode BCI is now 10.
The Synchron BCI is implanted in the blood vessel on the surface of the motor cortex of the brain via the jugular vein, through a minimally-invasive endovascular procedure. Once implanted, it is designed to detect and wirelessly transmit motor intent out of the brain, and intended to restore the capability for severely paralyzed people to control personal devices with hands-free point-and-click.
For more information about the Synchron BCI and our registry, visit https://synchronbci.com.
About Synchron
Synchron is a neurotechnology company developing an endovascular brain-computer interface (BCI) designed to restore functionality in people with severe paralysis. The clinical-stage company is developing a neuroprosthesis to restore motor signaling to control digital devices and autonomy for individuals with motor impairment. Synchron is headquartered in New York City. For more information, visit www.synchron.com. Follow us @synchroninc.
View source version on businesswire.com: https://www.businesswire.com/news/home/20240909176183/en/

 Business wire
Business wire 











Add Comment